
It is an inevitable law that the heat transfer oil will continue to deteriorate during use. The main reasons for the deterioration are high temperature, oxidation and impurities. The deterioration of the heat transfer oil not only reduces the heat transfer efficiency, but also endangers the safe operation of the equipment. Standardized use and maintenance of heat transfer oil Oil prolongs its service life, and can also be extended through regeneration.
1. Oxidation
Under the action of oxygen, the heat transfer oil is oxidized and converted into various oxides. The oxidation process is: under the action of molecular oxygen, hydrocarbons form free radicals and peroxides (reaction 1); free radicals combine with oxygen to form peroxyls (reaction 2); peroxyls react with hydrocarbons (reaction 3 ), forming a vicious circle. Its reaction formula is as follows:
Reaction 1: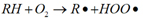
Reaction 2: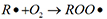
Reaction 3: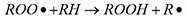
Therefore, in this reaction chain, the oxygen content and the activity of free radicals determine the reaction speed, and the activity of free radicals increases greatly with the increase of temperature. Oxidation rates also increase under the catalysis of metals and other impurities.
In the actual production process, it is necessary to avoid the existence of non-ferrous metals in the entire system. Due to their relatively active chemical properties, some non-ferrous metals can catalyze the free radical reaction of heat transfer oil oxidation at high temperatures; it is recommended to use a closed system and use an inert Gas or cold oil liquid seal device to block the pouring of oxygen atoms into the system.
2. Cracking and condensation
The main components of heat transfer oil are hydrocarbons. At high temperature, two types of chemical reactions mainly occur, one is cracking reaction and the other is condensation reaction. The molecular weight of the cracking reaction decreases, and the light components generated are discharged from the head tank; the molecular weight of the condensation reaction increases, forming colloids and asphaltenes, which are gradually separated from the heat transfer oil. Whether it is a cracking reaction or a condensation reaction, the produced substances will act as inducing factors in the heat transfer oil, inducing the heat transfer oil to continue to undergo cracking and condensation reactions. The generated asphaltenes are adsorbed on the metal pipe wall and heated to further dehydrogenate to form coke. In this reaction, high temperature and impurities will undoubtedly speed up the reaction.
In the actual production process, the appropriate type of manto heat transfer oil is selected according to the actual working temperature, so as to reduce the occurrence of cracking and polymerization caused by over-temperature, and avoid further vicious circles. Once cracking and polymerization occur, light components are removed in time Separation and removal of colloids can effectively inhibit the further deterioration of oil products.
3. The influence of impurities
The impact of impurities on the heat transfer oil is multifaceted. On the one hand, some impurities directly react with the heat transfer oil; on the other hand, some impurities, such as metals, can increase the activity of free radicals in the heat transfer oil and play a catalytic role. Accelerate the deterioration rate of heat transfer oil. Since some impurities are insoluble in the heat transfer oil, they continue to accumulate and precipitate in the heat transfer oil, and work together with insolubles such as asphaltene to adhere to the furnace tube wall to form a stagnant layer, which accelerates the coking speed and seriously affects the performance of the heat transfer oil.
In the actual production process, if there is too much insoluble matter in the oil, the operation of filtering and removing impurities in time can effectively prevent the deterioration of the oil and maintain the excellent performance of the heat transfer oil.